Intended purpose
In our consultation with manufacturers of medical devices, we repeatedly find that the terms "intended purpose," "normal use," and "intended use" are often used interchangeably. Admittedly, the terms are similar, and they are also related. But they are by no means synonyms. This article explains the difference. Intended purpose "[...] means the use for which a device is intended according to the information provided by the manufacturer on the label, in the instructions for use or in the promotional or sales material, or the advertising or sales claims and its statements in the clinical evaluation" (Article 2 No. 12 MDR / Article 2 No. 12 IVDR).
It is determined by the manufacturer and appears on
- the labeling of the product,
- the instructions for use and / or
- the promotional materials (including the website!!!).
I like to explain it like this: "The intended purpose is like a patent claim. This is where you specify what the product is supposed to be for, i.e. what its purpose is (as the name implies)."
But this has far-reaching consequences! Because the intended purpose forms the basis for (and this is not an exhaustive list):
- Classification as a medical device,
- Classification according to Annex VIII MDR,
- Clinical evaluation,
- Risk analysis,
- Proper application or use,
- product liability.
And with the penultimate point, we are already at the term "normal use".
Normal use
The term "normal use" is surprisingly not found in the MDR at all! It is defined in the DIN EN 60601-1 standard as follows: "3.71 Normal use: Operation, including routine checks and adjustments by the operator and standby in accordance with the instructions for use".
In medical device law, on the other hand, the terms "normal use" and "intended use" are used.
Since the term "normal use" is even more frequently used, I will also use this term in the following.
In any case, it becomes clear that the "normal use" is much more than the intended purpose!
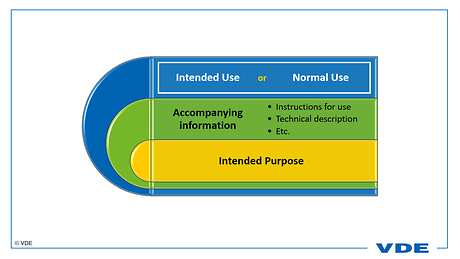
The basis for all considerations is the intended purpose. In addition, "normal use" includes the description of how the product is to be used! And of course also how the product is not to be used!
This information can be found in the accompanying documents, commonly the instructions for use, the technical description and other documents - we will deal with the topic of accompanying documents in another article. Note: "normal use" also refers to transport, storage, inspection, maintenance, etc.
The connection is clearly shown in the following graphic.